Arrange The Ions By Their Expected Hydration Energy 11+ Pages Summary in Doc [3mb] - Updated 2021
See 18+ pages arrange the ions by their expected hydration energy solution in Google Sheet format. Is the amount of energy released when one mole of ions undergo hydration ie. Arrange the ions by their expected hydration energy Highest hydration energy Lowest hydration energy Answer Bank K Ca. Rank these ions by their expected hydration energy. Read also their and arrange the ions by their expected hydration energy Ga 3 Ca 2 K.
The first part is the energy released when the solvent forms a coordination compound with the ions. Rank these ions according to ionic radius.

Arrange Na Mg 2 And Al 3 In Increasing Order Of Energy Of Hydration Rank these ions by their expected hydration energy.
| Topic: 12These properties result from the regular arrangement of the ions in the crystalline lattice and from the strong electrostatic attractive forces between ions with opposite charges. Arrange Na Mg 2 And Al 3 In Increasing Order Of Energy Of Hydration Arrange The Ions By Their Expected Hydration Energy |
| Content: Learning Guide |
| File Format: DOC |
| File size: 1.4mb |
| Number of Pages: 17+ pages |
| Publication Date: July 2021 |
| Open Arrange Na Mg 2 And Al 3 In Increasing Order Of Energy Of Hydration |
 |
The Mg 2 and Sr 2 ions have higher hydration energy.

Because of the smaller size of Al3 than M g2 and N a its energy of hydration larger than both these ions. Br - Sr2 Rb Se2- As3- Q. Highest hydration energy Lowest hydration energy Na Mg2 Al3. This energy released is called the Enthalpy of ligation Delta H_lig. Rank These Ions By Their Expected Hydration Energy Al3 Mg2 Na Question. Answer to Rank these ions by their expected hydration energy.
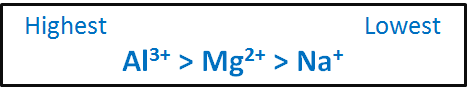
Emily S Studygram On Instagram Just Finished My Chemistry Exam And It Wasn T As Hard As I Expected It Now I Have 4 More Study Notes Exams Tips School Notes 15The hydration energy of the ions their coordination number and the structure of the water cluster coordinated with the ions were determined.
| Topic: The approach used to calculate ions properties was validated by comparing hydration properties provided by experimental and simulation works. Emily S Studygram On Instagram Just Finished My Chemistry Exam And It Wasn T As Hard As I Expected It Now I Have 4 More Study Notes Exams Tips School Notes Arrange The Ions By Their Expected Hydration Energy |
| Content: Solution |
| File Format: PDF |
| File size: 2.3mb |
| Number of Pages: 5+ pages |
| Publication Date: March 2018 |
| Open Emily S Studygram On Instagram Just Finished My Chemistry Exam And It Wasn T As Hard As I Expected It Now I Have 4 More Study Notes Exams Tips School Notes |
 |

Solution Rank These Ions Their Expect Chemistry A Smaller the size of the ion more highly it is hydrated and hence greater is the mass of the hydrated ion and thus the ionic mobility become lesser.
| Topic: While formation of ion pairs from isolated ions releases large amounts of energy even more energy is released when these ion pairs condense to form an ordered three-dimensional array. Solution Rank These Ions Their Expect Chemistry Arrange The Ions By Their Expected Hydration Energy |
| Content: Summary |
| File Format: Google Sheet |
| File size: 2.6mb |
| Number of Pages: 55+ pages |
| Publication Date: October 2017 |
| Open Solution Rank These Ions Their Expect Chemistry |
 |
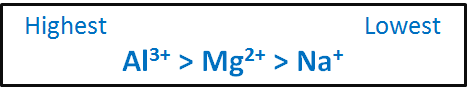
The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate 15The hydration energy of an ionic compound consists of two inseparable parts.
| Topic: Although this is an experiment that is impossible to perform the hydration energies have been determined indirectly you will see how this is done later in the term. The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate Arrange The Ions By Their Expected Hydration Energy |
| Content: Solution |
| File Format: PDF |
| File size: 1.7mb |
| Number of Pages: 21+ pages |
| Publication Date: July 2020 |
| Open The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate |
 |

Rank These Ions Their Expected Hydration Energy Chegg Rank these ions according to ionic radiusCa2 P3- S2- CI- K Q.
| Topic: Answer to Rank these ions by their expected hydration energy. Rank These Ions Their Expected Hydration Energy Chegg Arrange The Ions By Their Expected Hydration Energy |
| Content: Explanation |
| File Format: DOC |
| File size: 5mb |
| Number of Pages: 13+ pages |
| Publication Date: April 2019 |
| Open Rank These Ions Their Expected Hydration Energy Chegg |
 |

The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate Br - Sr2 Rb Se2- As3- Q.
| Topic: Because of the smaller size of Al3 than M g2 and N a its energy of hydration larger than both these ions. The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate Arrange The Ions By Their Expected Hydration Energy |
| Content: Learning Guide |
| File Format: PDF |
| File size: 2.2mb |
| Number of Pages: 7+ pages |
| Publication Date: November 2021 |
| Open The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate |
 |

Solution Rank These Ions Their Expect Chemistry
| Topic: Solution Rank These Ions Their Expect Chemistry Arrange The Ions By Their Expected Hydration Energy |
| Content: Analysis |
| File Format: DOC |
| File size: 810kb |
| Number of Pages: 6+ pages |
| Publication Date: July 2019 |
| Open Solution Rank These Ions Their Expect Chemistry |
 |

Membranes Free Full Text Perfluorosulfonic Acid Membranes Thermally Treated And Modified Dopants With Proton Acceptor Properties For Asparaginate And Potassium Ions Determination In Pharmaceuticals Html
| Topic: Membranes Free Full Text Perfluorosulfonic Acid Membranes Thermally Treated And Modified Dopants With Proton Acceptor Properties For Asparaginate And Potassium Ions Determination In Pharmaceuticals Html Arrange The Ions By Their Expected Hydration Energy |
| Content: Explanation |
| File Format: PDF |
| File size: 2.8mb |
| Number of Pages: 9+ pages |
| Publication Date: January 2021 |
| Open Membranes Free Full Text Perfluorosulfonic Acid Membranes Thermally Treated And Modified Dopants With Proton Acceptor Properties For Asparaginate And Potassium Ions Determination In Pharmaceuticals Html |
 |

Solution Rank These Ions Their Expect Chemistry
| Topic: Solution Rank These Ions Their Expect Chemistry Arrange The Ions By Their Expected Hydration Energy |
| Content: Synopsis |
| File Format: DOC |
| File size: 2.2mb |
| Number of Pages: 50+ pages |
| Publication Date: January 2018 |
| Open Solution Rank These Ions Their Expect Chemistry |
 |

Why Does Hydration Enthalpy Decrease On Going Down A Group Quora
| Topic: Why Does Hydration Enthalpy Decrease On Going Down A Group Quora Arrange The Ions By Their Expected Hydration Energy |
| Content: Summary |
| File Format: DOC |
| File size: 2.3mb |
| Number of Pages: 30+ pages |
| Publication Date: September 2021 |
| Open Why Does Hydration Enthalpy Decrease On Going Down A Group Quora |
 |

Solution Rank These Ions Their Expect Chemistry
| Topic: Solution Rank These Ions Their Expect Chemistry Arrange The Ions By Their Expected Hydration Energy |
| Content: Analysis |
| File Format: DOC |
| File size: 1.8mb |
| Number of Pages: 9+ pages |
| Publication Date: February 2020 |
| Open Solution Rank These Ions Their Expect Chemistry |
 |

Petitive Sorption Of Monovalent And Divalent Ions Highly Charged Globular Macromolecules The Journal Of Chemical Physics Vol 153 No 4
| Topic: Petitive Sorption Of Monovalent And Divalent Ions Highly Charged Globular Macromolecules The Journal Of Chemical Physics Vol 153 No 4 Arrange The Ions By Their Expected Hydration Energy |
| Content: Summary |
| File Format: DOC |
| File size: 1.4mb |
| Number of Pages: 20+ pages |
| Publication Date: October 2019 |
| Open Petitive Sorption Of Monovalent And Divalent Ions Highly Charged Globular Macromolecules The Journal Of Chemical Physics Vol 153 No 4 |
 |
Its really easy to prepare for arrange the ions by their expected hydration energy Emily s studygram on instagram just finished my chemistry exam and it wasn t as hard as i expected it now i have 4 more study notes exams tips school notes the enthalpy of hydration of the fe 2 ion is 11 4kcal mol higher than would be expected if there were no crystal field stabillisation energey assuming the equo plex to be high s estimate the enthalpy of hydration of the fe 2 ion is 11 4kcal mol higher than would be expected if there were no crystal field stabillisation energey assuming the equo plex to be high s estimate arrange na mg 2 and al 3 in increasing order of energy of hydration why does hydration enthalpy decrease on going down a group quora the enthalpy of hydration of the fe 2 ion is 11 4kcal mol higher than would be expected if there were no crystal field stabillisation energey assuming the equo plex to be high s estimate membranes free full text perfluorosulfonic acid membranes thermally treated and modified dopants with proton acceptor properties for asparaginate and potassium ions determination in pharmaceuticals html petitive sorption of monovalent and divalent ions highly charged globular macromolecules the journal of chemical physics vol 153 no 4

Post a Comment
Post a Comment